INTRODUCTION
MATERIALS AND METHODS
Table 1.
RESULTS
Table 2.
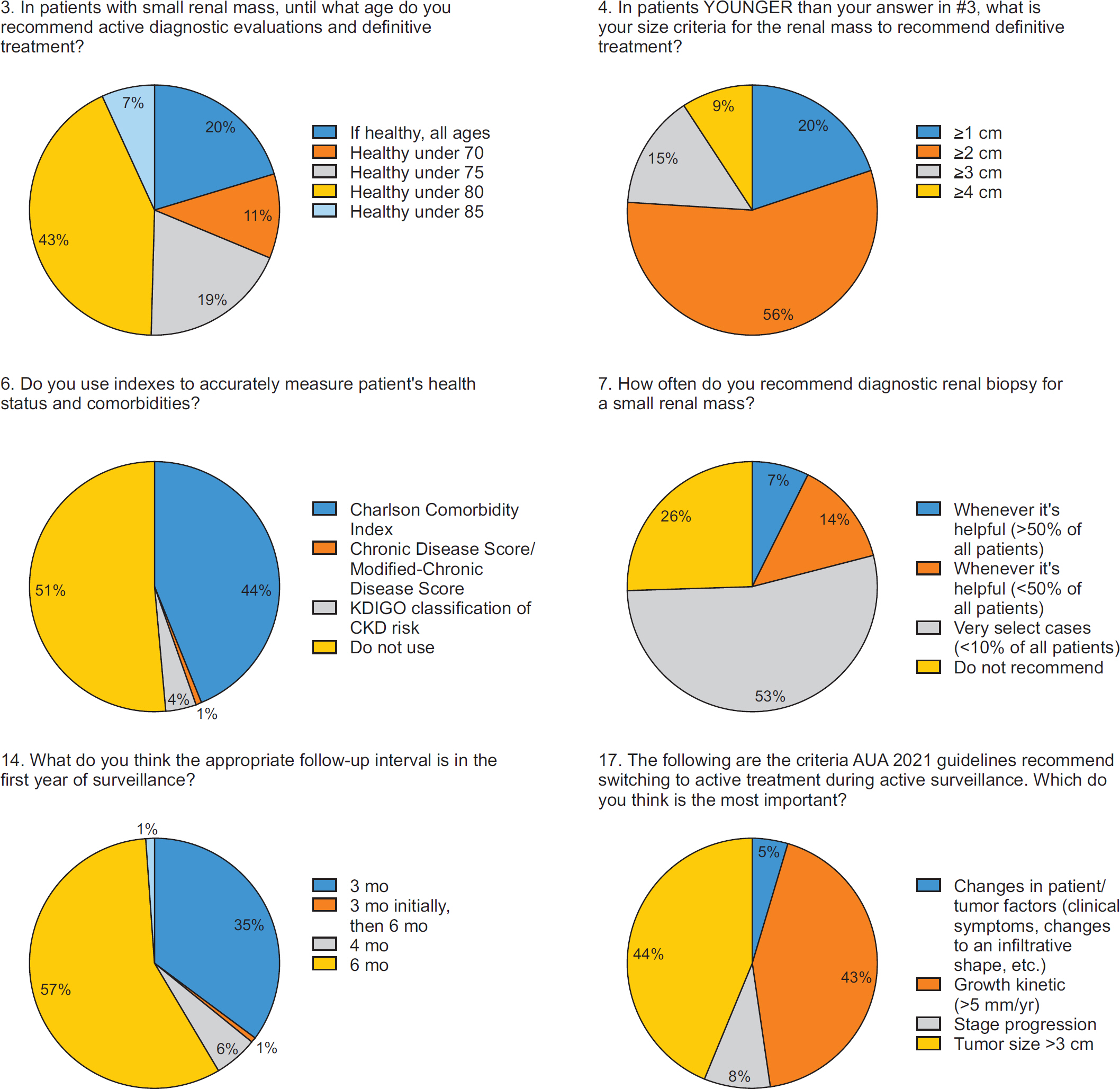
1. Age and Competing Risks
2. Size and Growth Kinetics
3. Termination of Surveillance and Intervention
Table 3.
4. Renal Mass Biopsy
DISCUSSION
Fig. 1.
| J Urol Oncol > Volume 21(1); 2023 > Article |
|


Joongwon Choi 
https://orcid.org/0000-0001-5978-8179
Cheryn Song 
https://orcid.org/0000-0002-1823-4281
Jungyo Suh 
https://orcid.org/0000-0002-3867-4778
Minyong Kang 
https://orcid.org/0000-0002-6966-8813
Chang Il Choi 
https://orcid.org/0000-0001-6488-1933
Hyeong Dong Yuk 
https://orcid.org/0000-0002-5874-9167
Chan Ho Lee 
https://orcid.org/0000-0002-7750-6806
Jung Kwon Kim 
https://orcid.org/0000-0002-8069-6225
Jung Ki Jo 
https://orcid.org/0000-0002-6080-7493
Won Sik Ham 
https://orcid.org/0000-0003-2246-8838
Eu Chang Hwang 
https://orcid.org/0000-0002-2031-124X
Chang Wook Jeong 
https://orcid.org/0000-0002-2200-5019
Young Hwii Ko 
https://orcid.org/0000-0002-9150-4292
Jae Young Park 
https://orcid.org/0000-0002-6664-6846
Seong Il Seo 
https://orcid.org/0000-0002-9792-7798
Byung Kwan Park 
https://orcid.org/0000-0002-4114-8859
Jinsoo Chung 
https://orcid.org/0000-0003-2251-5331
Cheol Kwak 
https://orcid.org/0000-0002-1987-2111
Sung-Hoo Hong 
https://orcid.org/0000-0002-1952-4010