| J Urol Oncol > Volume 21(2); 2023 > Article |
|
Abstract
Purpose
Materials and Methods
Results
NOTES
Fig. 1.

Fig. 2.
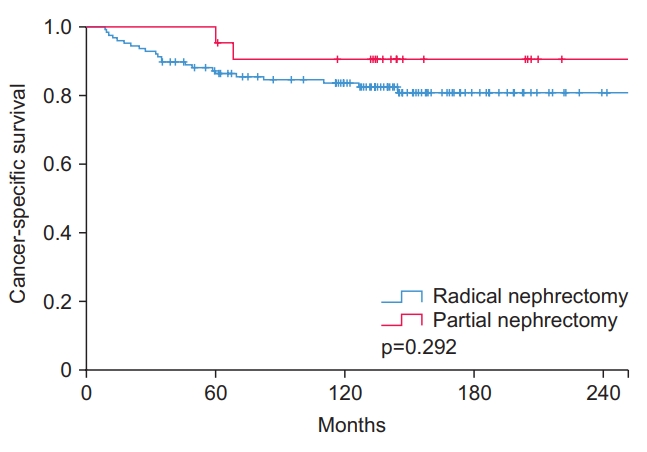
Table 1.
| Characteristic | Overall | PN | RN | p-value |
|---|---|---|---|---|
| No. of patients (%) | 149 (100) | 22 (14.8) | 127 (85.2) | |
| Age (yr) | 56.5±12.0 | 61.1±9.8 | 55.7±12.2 | 0.050 |
| Sex | 0.218 | |||
| Male | 98 (65.8) | 17 (77.3) | 81 (63.8) | |
| Female | 51 (34.2) | 5 (22.7) | 46 (36.2) | |
| BMI (kg/m2) | 24.7±3.2 | 24.6±2.9 | 24.7±3.3 | 0.765 |
| Surgical approach | 0.094 | |||
| Open | 84 (56.4) | 16 (72.7) | 68 (53.5) | |
| Laparoscopic, robotic | 65 (43.6) | 6 (27.3) | 59 (46.5) | |
| Tumor laterality | 0.381 | |||
| Right | 60 (40.3) | 7 (31.8) | 53 (41.7) | |
| Left | 89 (59.7) | 15 (68.2) | 74 (58.3) | |
| Tumor size (cm) | 6.4±3.1 | 3.1±1.7 | 7.0±2.9 | <0.001* |
| Clinical stage† | <0.001* | |||
| T1a | 26 (17.4) | 15 (68.2) | 11 (8.7) | |
| T1b | 29 (19.5) | 1 (4.5) | 28 (22.0) | |
| T2a | 19 (12.8) | NA | 19 (15.0) | |
| T2b | 3 (2.0) | NA | 3 (2.4) | |
| T3a | 72 (48.3) | 6 (27.3) | 66 (52.0) | |
| Reason for pT3 | 0.004* | |||
| Perinephric fat invasion | 80 (53.7) | 18 (81.8) | 62 (48.8) | |
| Renal sinus invasion | 69 (46.3) | 4 (18.2) | 65 (51.2) | |
| RCC Histological subtype | 0.444 | |||
| Clear cell | 121 (81.2) | 17 (77.3) | 104 (81.9) | |
| Papillary | 5 (3.4) | 2 (9.1) | 3 (2.4) | |
| Chromophobe | 14 (9.4) | 2 (9.1) | 12 (9.4) | |
| Others | 9 (6.0) | 1 (4.5) | 8 (6.3) | |
| Fuhrman nuclear grade | 0.098 | |||
| 1 | 2 (1.3) | 1 (4.5) | 1 (8.0) | |
| 2 | 40 (26.8) | 9 (40.9) | 31 (24.4) | |
| 3 | 82 (55.0) | 11 (50.0) | 71 (55.9) | |
| 4 | 25 (16.8) | 1 (4.5) | 24 (18.9) | |
| Resection margin positive | - | NA | NA |
Table 2.
HR, hazard ratio; CI, confidence interval; PN, partial nephrectomy; RN, radical nephrectomy; BMI, body mass index; Open, open nephrectomy; Lapa, laparoscopic nephrectomy; Robot, robot-assisted nephrectomy; Perinephric, perinephric fat invasion; Sinus, renal sinus invasion; Clear cell, clear cell renal cell carcinoma; Non-clear cell, non-clear cell renal cell carcinoma.
Table 3.
HR, hazard ratio; CI, confidence interval; PN, partial nephrectomy; RN, radical nephrectomy; BMI, body mass index; Open, open nephrectomy; Lapa, laparoscopic nephrectomy; Robot, robot-assisted nephrectomy; Perinephric, perinephric fat invasion; Sinus, renal sinus invasion; Clear cell, clear cell renal cell carcinoma; Non-clear cell, non-clear cell renal cell carcinoma.
REFERENCES
-
METRICS

-
- 1 Crossref
- 0 Scopus
- 853 View
- 25 Download
- ORCID iDs
-
Dongsu Kim

https://orcid.org/0000-0002-1960-9495Bumjin Lim

https://orcid.org/0000-0002-1746-6072Jungyo Suh

https://orcid.org/0000-0002-3867-4778Dalsan You

https://orcid.org/0000-0001-8152-847XCheryn Song

https://orcid.org/0000-0002-1823-4281In Gab Jeong

https://orcid.org/0000-0003-4093-832XBumsik Hong

https://orcid.org/0000-0003-1991-1229Jun Hyuk Hong

https://orcid.org/0000-0003-2705-0481Hanjong Ahn

https://orcid.org/0000-0001-7608-5352 - Related articles
-
The Role of Immunotherapy in the Era of TKI with Metastatic Renal Cell Carcinoma2014 August;12(2)
Role of Lymphadenectomy in Renal Cell Carcinoma2008 August;6(2)
Pararenal Pseudocyst Accompanied by Renal Cell Carcinoma2008 April;6(1)


 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print